What are solids
Solids are chemical substances which are characterized by definite mass, shape and volume, rigidity, high density, low compressibility. The constituent particles (atoms, molecules or ions) are closely packed and held together by strong intermolecular forces
General characteristics of solids: –
(1) Solids have definite mass, shape and volume.
(2) The intermolecular distance is minimum in solids and intermolecular forces are strong.
(3) The constituent particles i.e., atoms, molecules or ions have fixed positions.
(4) Solids cannot be compressed except foam, rubber, sponge, etc.
(5) Most of them have high melting and boiling point.
(6) Solids are rigid
Types of solids:
There are two types of solids.:- Crystalline Solids and Amorphous Solids
(1) Crystalline solids:
The solids in which the constituent particles have an ordered arrangement (long range order) are crystalline solids. For example, Sodium chloride, Diamond, Iodine, etc.
Characteristics of Crystalline solids
(i) Constituent particles are regularly arranged.
(ii) They possess strong melting point.
(iii) Their outer surface also shows a regular arrangement during the formation of crystals.
(iv) These are anisotropic in nature.
(v) Crystalline solids have a definite geometric shape with flat faces and sharp edges.
(vi) Crystalline solids have long range order of arrangement of constituent particles
Classification of Crystalline solids: On the basis of binding forces, crystalline solids are classified as follows:
(i) Molecular solids: – Constituent Particles are molecules. These are further divided in into three categories
(a) Non-polar molecular solids:
|
i |
Constituent particles |
Non-polar molecules |
|
Ii |
Bonding force |
Dispersion forces or London forces |
|
iii |
Binding energy in (kJ/mol) |
0.05-40 |
|
iv |
Melting point |
Very low about 84 |
|
v |
Physical nature |
Soft |
|
vi |
Electrical conductivity |
Insulator |
|
|
Examples |
H2, N2, O2, He, NA Ar, Kr |
(b) Polar molecular solids:
|
i |
Constituent particles |
Polar molecules |
|
Ii |
Bonding force |
Dipole -dipole interaction |
|
iii |
Binding energy in (kJ/mol) |
5-25 |
|
iv |
Melting point |
low about 158 |
|
v |
Physical nature |
Soft |
|
vi |
Electrical conductivity |
Insulator |
|
|
Examples |
HCl, HBr, SO2, SO3 etc |
(c) H-bonded molecular solids:
|
i |
Constituent particles |
Polar molecules containing O, N F and H |
|
Ii |
Bonding force |
Hydrogen bonding and Dipole -dipole interaction |
|
iii |
Binding energy in (kJ/mol) |
10-40 |
|
iv |
Melting point |
low about 273 |
|
v |
Physical nature |
Hard |
|
vi |
Electrical conductivity |
Insulator |
|
|
Examples |
H2O (Ice) |
(ii) Ionic solids: – 2. Constitute particles are Ions (positive and negative). Attractive forces are Coulombic or Electrostatic. For example, NaCl, MgO, ZnS, CaF2 Hard but do not conduct electricity in solid state but conduct in molten state and in aqueous solutions
(iii) Metallic Solids: – Constitute particles are atom. Attractive forces are metallic forces. For example. They conduct electricity in solid and molten state. for example, Al, Fe, Sn, Zn etc.
(iv) Covalent solids: – Constituent particles are Atoms and Bonding forces are Covalent bonds. They are very hard and having high melting point. Insulator and non-conductor of heat except Graphite. Example SiO2, SIO2, diamond, graphite, SiC (Carborundum)
(2) Amorphous solids: –
Solids in which constituents do not possess the orderly arrangement over the long range are called amorphous solids. Region in an amorphous solid having an orderly arrangement are known as crystallite. They may have only short-range order and do not possess sharp melting points. They undergo irregular cleavage (cut). Structures of amorphous solids are similar to that of liquid. Amorphous solids soften over range of temperature and can be molded and blown in to various shapes. On heating they become crystalline at some temperature. Therefore, some time they are called as pseudo solids or super cooled liquids Glass become milky sometimes on heating is due to this property. Due to lack of long-range arrangement of particles or irregular arrangement of particles, amorphous solids are isotropic in nature. It is physical properties like resistivity. Refractive index is independent of direction
Examples: Glass rubber and plastics are typical examples of amorphous solids. Structure of quartz is crystal while quartz glass is amorphous. Amorphous silicon is one of the best photovoltaic materials available for conversion of sunlight to electricity.
These have a gradual (range) of melting point. For example, Plastic, Glass, Anthracene etc.
Characteristics of Amorphous solids:
(i) Constituent particles are not arranged regularly and are irregular.
(ii) These solids do not show sharp melting point.
(iii) Outer surface does not show regular arrangement during the formation of crystals.
(iv) These are isotropic in nature.
(v) They have short range order of arrangement of constituent particles
Isotropy and Anisotropy:
Solids whose value of any physical property (Thermal conductivity, heat capacity, refractive index, electrical conductivity) would be same along any direction. This property is called isotropy. Amorphous solids are isotropic in nature. Reason of isotropy is same number of particles in all directions
The solids whose some of the physical properties like electrical resistance or refractive index show different values when measured in different directions in the same crystal, this property is called anisotropy. Crystalline solids are anisotropic in nature. Reason of Anisotropy is different number of particles in different directions
Polymorphic forms or polymorphs:
The different crystalline forms of a substance are known as polymorphic forms or polymorphs. For example: graphite and diamond.
Crystal lattice:
The regular three-dimensional arrangement of the constituent particles in a crystal in which each particle is represented by a point is called a crystal lattice and points are called lattice points.
Unit cell:
It is the smallest, fundamental repeated three-dimensional unit of a crystal lattice
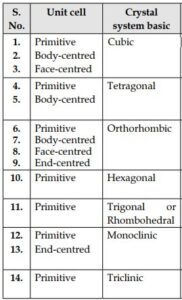
Types of unit cells:
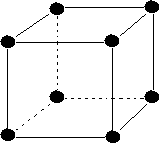
(i) Simple or primitive:- The unit cells in which, particles (i.e. atoms ions, or molecules) are present only at the corners of the unit cell are called simple or primitive cells
(ii) Face-centered:- In this type of unit cells, points are represented at the corners as well as centers of each six faces
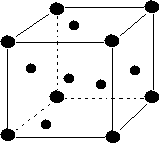
(iii) Body-centered:-These are the unit cells in which points are present at thecorners and an additional point is presentat the center of the unit cell
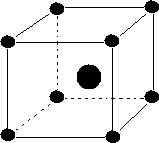
(iv) End centered:- The unit cell in which points are present at the corners and at the center of the two ends faces
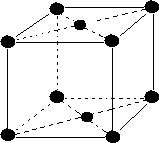
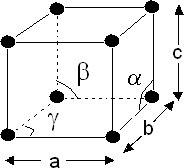
Crystal system and Bravais Lattice:
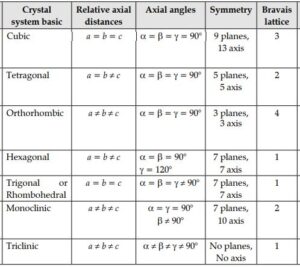
On the basis of parameter of edge length (a, b, c) and bond angle between them (α, β, γ) there are seven types of unit cells.
It should be noted that
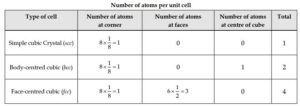
(i) An atom present at the corner is equally shared by eight-unit cells. Therefore, contribution of an presentat the corner to each unit cell is 1/8
(ii) An atom presentat the face center is equally sharedbetween two-unit cells. Therefore, contribution of an atom present at the face center towardseach unit cell is ½
(iii) An atom present within the body of the unit cell (body center) is shared by no other unit cell. Hence, contribution of an atom present withinbody of unit cell is 1.
(iv) An atom present at the edge centre of unit cell is equally shared by four unit cells. Therefore, contribution of an atom present at the edge centre towards each unit cell is ¼
Closed-packed structures:
Types of close packing:
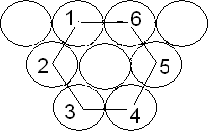
Close packing in one dimension: When the spheres representing particles are touching each other in a row, it is called close packing
Closed packing in two dimensions: This type of packing is obtained by placing the rows of close-packed spheres. This can be done in two ways:
Close-packing in three dimensions:
All solids exist in three dimensional structures. These structures can be obtained by placing two dimensional layers one above other. They can be of two types:
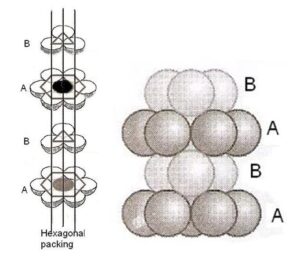
(i) Hexagonal close packed layers: In this arrangement, atoms are located at the corners and center of two hexagonal placed parallel to each other , three more atoms are placed in a parallel to midway between these two planes:
Characteristic features of hcp
(i) This type of packing is ABAB… type of arrangement of the layers which indicates that every alternate layers are alike
(ii) It has a 6-fold axis of symmetry
(iii) Each atom is surrounded by 12 another atoms, 6 in own layer and 3 above and 3 blow layers. Coordination number 12
(iv) In hcp arrangement, the atom occupy 74% of the available space and thus 26% of space is empty
(v) It has only one set of parallel close –packed layers. Hence, the chances for slipping of one layer over the other is less..
Example : BE, Cd, Li, Ca, Cr, Mo, V, Mg, Zn, Ce, Zr, OS, Ru, He
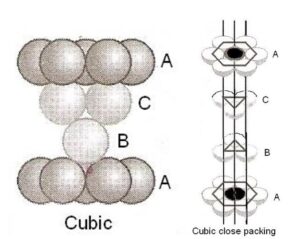
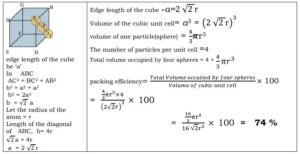
(ii) C ubic closed packing (ccp) or face centered cubic (fcc)
In this type of close packing, atoms are arranged at the corners and at the centers of all six facesof a cube.
If we start with hexagonal layersof spheres as shown in figure and second layer of spheresis arranged placing the spheres over the holes in first layer, one half of the holes can be filled by these spheres. Suppose that spheres in third layers are so arranged that they cover holes in second layer. , the third layer neither resembles first layer or second layer. The fourth layer resembles first , fifth resembles second and sixth resembles third layer, then this type of arrangement is known as cubic closed-packed (ccp) arrangement or face centered cubic (fcc) arrangement. The percentage of free space is 26% and coordination number is 12.
Characteristic featuresof ccp:
(i) This type of packing has ABCABC…. type of arrangement of the layer i.e. the first threelayers are quite different from each other but this set of layer is repeated over and again the additionof more layers
(ii) It has cubic symmetry,the whole structure has four 3-foldaxis of symmetry
(iii) As in hcp, each atom in ccp arrangement has 12 nearesthas 12 nearest neighbors i.e. the coordinate number of each atom is 12
(iv) The ccp arrangement of atoms occupies 74% of the available space and thus 26% of the space remains empty.
(v) It has four sets of parallel close packed layers. Hence, the chances for slipping of one layer over the other are more in the ccp arrangement than in the hcp arrangement. Hence, metals having ccp structure
(vi) Example : Cu, Ag, Au, Pt, Al, Cr, Co, Cu, Ag, Fe, PB, Mn, Ni, Ca, Sr, Pt all noble gases except He are found to posses cubic close packed structure. Nearly 60% of the metals have been found to possesseither hcp or ccp structure.
Body – Centered cubic structure ( bcc)
(i) In a body centered cubic arrangement, the atoms occupycorners of a cube with an atom at its centre.
(ii) Each atom is in contactwith eight other atoms ( four atoms in thelayer just above and four atoms in the layer just below) and hence the coordination number in this type ofarrangement is only eight.
(iii) This arrangement of atoms occupies only 68% of the total volume, so this arrangement is found in Na, K, Cs, Rb, W, V, Mo, and Ba. Only 20% of the metallic elements found toposses bcc arrangement.
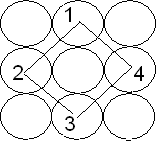
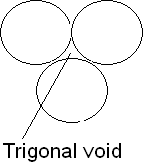
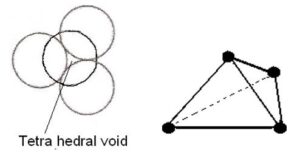
Trigonal voids: The void enclosed by three spheres in contact is called a trigonal void. There are 24 voids around each sphere. There are eight trigonal voids per atom in a crystal. Radius ratio is 0.155
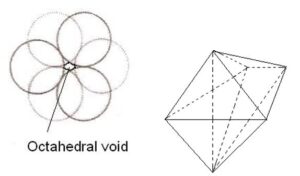
Tetrahedral voids: The void surrounded by four sphere lying at the vertices of a regular tetrahedron is called tetrahedral void. There are 8 tetrahedral voids around each sphere and two voids per atom of crystal. So, number of tetrahedral voids = 2 × Number of close packed spheres = 2N. The radius of a tetrahedral void in a closest packed arrangement is 22.5% of the sphere involved in this arrangement. Thus, R(void)/R(sphere)= 0.225
This type of void is formed between 8 closely packed spheres which occupy all eight corners of cube. This site is surrounded by eight sphereswhich touches each other. Size of the cubical void is given as If r =radius of the spherical cubical site R= radius of closely packed sphere
1. Decreasing orderof the size of the various voids : Cubic > Octahedral > Tetrahedral >Trigonal
2. Anion always present in packing
Cations always occupy the voids
Number of particles in packing (n) =
no. of octahedral voids = n
Number of tetrahedral voids = 2x no. of octahedral void
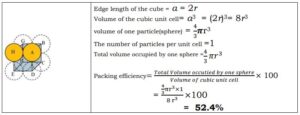
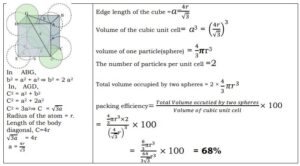
Packing Efficiency and Relation between edge length(a) and radius of sphere(r)
Radius Ratio
|
|
Density of Solid:-
It is also known imperfection in solids. Ideal crystal is that which has definite repeating arrangement of particles of atoms in crystal. Any departure from perfectly ordered arrangement of atom in crystal is called defect or imperfection.
Mainly there are two types of defects: – (1) Point defect. (2) Line defect.
Point defect: This defect is also known as atomic imperfections. When deviations exist from the regular arrangement around a point or an atom in a crystalline substance, the defect is called point defect
Types of point defects: Point defects are classified into three types:
(a) Stoichiometric defects
(b) Impurity defects
(c) Non-Stoichiometric defects
(a) Stoichiometric defects: Defects which do not change the stoichiometry of solids are called stoichiometric defects. These are also intrinsic or thermodynamic defects. There are four types of this defect:
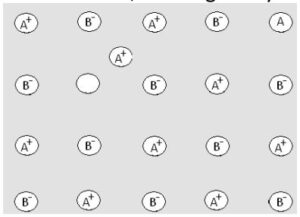
(i) Vacancy defect:When some lattice sites in a crystal line solid are vacant, then crystal is said to have vacancy defect. This defect arises on heating. As a result, the density of the solid decreases.
(ii) Interstitial defect:When some extra constituent particles occupy the interstitial site in crystal, defect is known as interstitial defect. It arises by applying high pressure on the crystal. This defect increases the density of the crystal. Both these defects are shown by non-ionic solids.
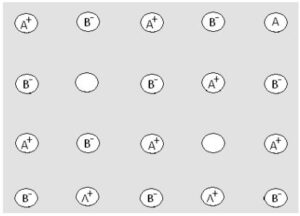
(iii) Frenkel defects:It is a type of vacancy defect. In ionic compounds, some of the ions (usually smaller in size) get dislocated from their original site and create defect. This defect is known as Frenkel Defects. Since this defect arises because of dislocation of ions, thus it is also known as Dislocation Defects. As there are a number of cations and anions (which remain equal even because of defect); the density of the substance does not increase or decrease. Ionic compounds; having large difference in the size between their cations and anions; show Frenkel Defects, such as ZnS, AgCl, AgBr, AgI, etc. These compounds have smaller size of cations compared to anions.
Consequences of Frenkel defect:l Crystal becomes conductor of electricity. l Stability of crystal decreases. l Dielectric constant increases.
(iv) Schottky defect:Schottky Defect is type of simple vacancy defect and shown by ionic solids having cations and anions; almost similar in size, such as NaCl, KCl, CsCl, etc. AgBr shows both types of defects, i.e. Schottky and Frenkel Defects
Consequences of Schottky defect: l Density of crystal decreases. l Lattice energy of crystal decreases. l Stability of crystal decreases. l Crystal becomes conductor of electricity. When cations and anions both are missing from regular sites, the defect is called Schottky Defect. In Schottky Defects, the number of missing cations is equal to the number of missing anions in order to maintain the electrical neutrality of the ionic compound.
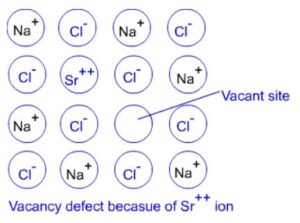
(b) Impurity defects: SrCl2 or CaCl2 is added to molten NaCl and it is crystallized. Some of the Na+ ions are replaced by Sr2+ or Ca2+ ions. Each Sr2+ or Ca2+ ions replace two Na+ ions. It occupies the site of one of the ions and other site remains vacant. The number of cationic vacancies are equal to the number of bivalent cations added. Similar example of impurity defect is addition of CdCl2 to AgCl.
(c) Non-stoichiometric defects: Those defects which lead to change in composition of solids are called non-stoichiometric defects. These defects are of two types: (i) Metal excess defect, (ii) Metal deficiency defect.
(i) Metal excess defect:This defect arises due to anionic vacancies or due to the presence of extra cation in the interstitial sites. The anionic sites occupied by unpaired electrons. This defect occur in the following ways:
Ø Metal excess defect due to anion vacancies:In this defect, negative ion from the crystal lattice may be missing from its lattice site leaving a hole or vacancy which is occupied by the electron originally associated with anion. In this way crystal remains neutral. Alkali halides like NaCl and KCl show this type of defect
Ø F Centres: These are anionic sites occupied by unpaired electrons. F– centres impart colour to crystals. The colour results by the excitation of electrons when they absorb energy from the visible light falling on the crystal. For example NaCl becomes yellow in colour when heated with sodium vapours. LiCl becomes pink, KCl becomes violet
Ø Metal excess defect due to interstitial cation:In this defect, an extra cation occupies interstitial posittion in the lattice and the free electron is trapped in the vacancy (vicinity) of this interstitial cation, but crystal remains neutral. For example: Zinc oxide on heating loses oxygen and turn yellow. ZnO heat
ZnO → Zn2+ + ½ O2+ 2e–
The excess of Zn2+ ions move to interstitial sites and the electrons to neighboring interstitial site.
(ii) Metal deficiency defect:In this, a cation is missing from its lattice site. To maintain electrical neutrality, one of the nearest metal ion acquires two positive charges. This type of defect occurs in compounds where metal can exhibit variable valency. e.g., transition metal compounds like NiO, FeO, FeS, etc
PROPERTIES OF SOLIDS (CRYSTALS)
The properties are solid depends on their composition, lattice structure and the nature of bond. Some properties of solid are as follows 1) Electrical properties 2) Magnetic properties 3) Dielectric properties
1) Electrical properties:
Solids may be classified into three categories depending upon their values of electric conductivity.
Conductors: electrical conductivity = 104 to 107 ohm-1 cm-1
Insulators: electrical conductivity = 10-20 to 10-10 ohm-1cm-1
Semiconductors: electrical conductivity = 10-6 to 104 ohm-1 cm-1
Causes of conductance in solid
(i) In most of the solids, conduction is through electron movement under an electric field
(ii) In ionic solids conduction is by movement of ions
(iii) The magnitude of electrical conductivity strongly depends upon the number of electrons available to take part in conduction process.
(iv) In metals, conductivity strongly depends upon the number of electrons available per Atom
(v)The atomic orbitals form molecular orbitals which forms band conduction band and valance band
(vi) In a case of metals conduction band and valance bands are to close or overlap and electrons flow very easily under electric field showing conductivity
(vii) gap between conduction band and valance band is known as forbidden gap
(viii) In case of insulators, the gap between valance band and conduction band is too large, so electrons cannot jump from valence band to conduction band and very small conductivity is observed.
(ix) In case of semiconductors, the gap between valance band and conduction band is small and therefore some of the electrons may jump from valance band and some conductivity is observed
(x) electrical conductivity of semiconductors increases, with increase in temperature. This is due to the fact that with increase in temperature, large number of valence electrons from valance band can jump to conduction band. Pure substances like Silicon and Germanium that exhibit this type conducting behavior are called intrinsic semiconductor.
Conduction in semiconductors
Conductivity of semiconductors can be increased by the introduction of impurity in semiconductors is called doping.
n-type semiconductors: When a silicon crystal is doped with a group 15 elements such as P, As, Sb, Bi, structure of crystal lattice is left unchanged but dopant atom with five valance electrons occupy the site normally occupied by silicon atom The foreign atom (dopant) uses four of its electrons for covalent bonding but fifth electron becomes delocalized and if thus free to contribute to electrical conduction. Silicon doped with group 15 element is called n-type semiconductor. ‘n’ stands for negative since electrons are responsible for conduction.
p-type semiconductors: – When a silicon crystal is doped with a group 13 elements such as B, Al, Ga, In, structure of crystal lattice is left unchanged but dopant atom with three valance electrons occupy the site normally occupied by silicon atom The foreign atom (dopant) uses three of its electrons for covalent bonding but fourth electron is missing is called an electron vacancy or hole. Such holes can move through the crystal-like positive charge giving rise to electrical conductivity. Direction of motion of holes is opposite to that of electron. Silicon doped with group 13 element is called p-type semiconductor. ‘p’ stands for positive since electrons are responsible for conduction.
13-15 compounds: When the solid-state materials are produced by combination of elements of groups 13 and 15, the compounds thus obtained are called 12-16 compound. Example InSb, AlP, GaAs
12-16 Compound. When the solid-state compounds are obtained by combination of elements of group 12 and 16, the compounds are called 12-16 compounds. Example ZnS, CdS, CdSe and HgTe Transition metal oxides. Some transition metal oxide like TiO, CrO, ReO3 behaves like metals. ReO3 behaves like copper in appearance as well as conductance. VO, VO2 VO3 and TiO3 also show electrical conductance depending on temperature.
MAGNETIC PROPERTIES:
Substance shows magnetic properties because of presence of electrons in them. Each electron in an atom behaves like a magnet because of its two types of motions – one is around their axis and other around the nucleus. Electrons in an atom because of charge over then and in motion continuously; possess small loop of current which shows the magnetic moment. Substances are classified into five types on the basis of magnetic properties:
a. Paramagnetic
b. Diamagnetic
c. Ferromagnetic
d. Antiferromagnetic
e. Ferrimagnetic
(a) Paramagnetism: Substances which are attracted slightly by magnetic field and do not retain the magnetic property after removal of magnetic field are called paramagnetic substances. electrons are randomly aligned with respect to applied ,magnetic field For example O2, Cu2+, Fe3+, Cr3+, Magnesium, molybdenum, lithium, etc. 21 Substances show Paramagnetism because of presence of unpaired electrons. These unpaired electrons are attracted by magnetic field.
↗ ↑ ↖ ↑ ↗ ↑
(b) Diamagnetism: Diamagnetic substances are just opposite to that of paramagnetic. Substances which are repelled slightly by magnetic field are called diamagnetic substances. For example; H2O, NaCl, C6H6, etc. Diamagnetic substances are magnetized slightly when put under magnetic field but in opposite direction.
Substances show diamagnetic property because of presence of paired electrons and no unpaired electron. Thus, pairing of electrons cancel the magnetic property
↑↓ ↑↓ ↑↓ ↑↓
(c) Ferromagnetism: Substances that are attracted strongly with magnetic field are called ferromagnetic substances, such as cobalt, nickel, iron, gadolinium, chromium oxide, etc. Ferromagnetic substances can be permanently magnetized also. Metal ions of ferromagnetic substances are randomly oriented in normal condition and substances do not act as a magnet. But when metal ions are grouped together in small regions, called domains, each domains act like a tiny magnet and produce strong magnetic field, in such condition ferromagnetic substance act like a magnet. When the ordering of domains in group persists even after removal of magnetic field a ferromagnetic substance becomes a permanent magnet.
↑ ↑ ↑ ↑ ↑
(d) Antiferromagnetism: Substances in which domain structure are similar to ferromagnetic substances but are oriented oppositely, which cancel the magnetic property are called antiferromagnetic substances and this property is called Antiferromagnetism. Or in simple words equal number of parallel and anti-parallel alignment of unpaired electron. For example; MnO.
↑ ↓ ↑ ↓ ↑ ↓
(e) Ferrimagnetism: Substances which are slightly attracted in magnetic field and in which domains are grouped in parallel and anti-parallel direction but in unequal number, are called ferromagnetic substances and this property is called ferrimagnetism. For example, magnetite (Fe3O4), ferrite (MgFe2O4), ZnFe2O4, etc.