Metallurgy
Metallurgy:- It is the Conversion of Metal ores into Pure metal
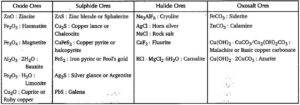
Ores:- From which extraction of metal is easy, profitable and ecofriendly.
A. Crushing and grinding:
B. Concentration:-
C. Extraction:-
D. Refining :-
A. Crushing and grinding: The first process in metallurgy is crushing of ores into a fine powder in a crusher or ball mill. This process is known as pulverization.
B. Concentrations:-
The process of removal unwanted materials like sand, clay, rocks etc from the ore is known as concentration, ore – dressing or benefaction. It involves several steps which depend upon physical properties of metal compound and impurity (gangue). The type of metal, available facilities and environmental factors are also taken into consideration .
Concentration is of following types:-
1. Hydraulic washing,
2. Magnetic separation,
3. Froth Flotation process,
4.Leaching
1. Hydrolytic method: Hydraulic washing (or gravity separation): It is based on difference in densities of ore and gangue particles. Ore is washed with a stream of water under pressure so that lighter impurities are washed away whereas heavy ores are left behind.
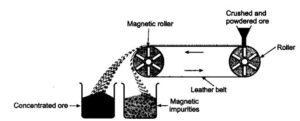
2. Magnetic separations:- This method is based on the difference in magnetic and non – magnetic properties of two components of ore (pure and impure). This method is used to remove tungsten ore particles from cassiterite (SnO2). It is also used to concentrate magnetite (Fe3O4), chromite (FeCr2O4) and pyrolusite (MnO2) from unwanted gangue
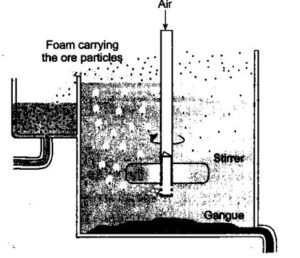
3. Froth floatation: This method is based on the difference in magnetic and non – magnetic properties of two components of ore (pure and impure). This method is used to remove tungsten ore particles from cassiterite (SnO2). It is also used to concentrate magnetite (Fe3O4), chromite (FeCr2O4) and pyrolusite (MnO2) from unwanted gangue enhance the non-wettability of the mineral particles. Examples are pine oil, fatty acids and xanthates are added to it. Froth stabilizers are added to stabilize the froth. Examples are cresols, aniline. If two sulphide ores are present, it is possible to separate the two sulphide ores by adjusting proportion of oil to water or by adding depressants. For example- In the case of an ore containing ZnS and PbS, the depressant used is NaCN. It selectively prevents ZnS from coming to froth but allows PbS to come with the froth
4. Leaching (Chemical separation): It is a process in which ore is treated with suitable solvent which dissolves the ore but not the impurities
C. Extraction :- It occurs in two steps:
1 Roasting and calcination: It is a process in which ore is heated in a regular supply of air at a temperature below melting point of the metal so as to convert the given ore into oxide ore. By this method Sulphide ores are converted into oxide. It is also used to remove impurities as volatile oxides E.g.: 2ZnS+ 3O2 → 2ZnO + 2SO2.
Calcination is a process of heating ore in limited supply of air so as to convert carbonate ores into oxides.CBy this method Carbonate ores are converted into oxide by roasting. t is also used to remove moisture and volatile impurities E.g.: CaCO3 ——–> CaO CO2
(b).Reduction of oxide to metal: The process of converting metal oxide into metal is called reduction. It needs a suitable reducing agent depending upon the reactivity or reducing power of metal. The common reducing agents used are carbon or carbon monoxide or any other metals like Al, Mg etc.
Also Watch
Thermodynamic principles of metallurgy: Some basic concepts of thermodynamics help in understanding the conditions of temperature and selecting suitable reducing agent in metallurgical processes:
(I) . Gibbs free energy change at any temperature is given by ΔG = ΔH – TΔS where ΔG is free energy change, ΔH is enthalpy change and ΔS is entropy change.
(ii). The relationship between ΔGӨ and K is ΔGӨ = –2.303 RT log K where K is equilibrium constant. R = 8.314 JK- ¹ mol-1, T is temperature in Kelvin.
(iii) A negative ΔG means +ve value of K i.e., products are formed more than the reactants. The reaction will proceed in forward direction.
(iv). If ΔS is +ve, on increasing temperature the value of TΔS increases so that TΔS > ΔH and ΔG will become negative
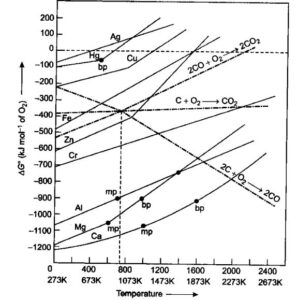
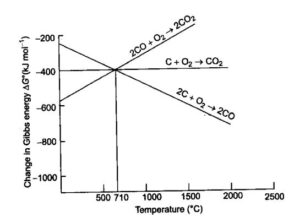
Ellingham diagrams: The plots between ∆fGо of formation of oxides of elements vs. temperature are called Ellingham diagrams. It provides a sound idea about selecting a reducing agent in reduction of oxides. Such diagrams help in predicting the feasibility of a thermal reduction of an ore. ΔG must be negative at a given temperature for a reaction to be feasible
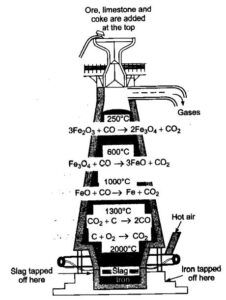
Reduction of iron oxide in blast furnace: Reduction of oxides takes place in different zones.
- At 500 – 800 K (lower temperature range in blast furnace)
- At 900 – 1500 K (higher temperature range in blast furnace
Reduction of Iron from its oxide is easier by CO at lower temperature, Whereas at high temperature by C Because of less Standard Gibb’s Free energy.
Types of iron:
Pig iron: The iron obtained from blast furnace is called pig iron. It is impure from of iron contains 4% carbon and small amount of S,.P, Si and Mn. It can be casted into variety of shapes.
Cast iron: It is made by melting pig iron with scrap iron and coke using hot air blast. It contains about 3% of carbon content. It is extremely hard and brittle.
Wrought iron: It is the purest form of commercial iron. It is also called malleable iron. t is prepared by oxidative refining of pig iron in reverberatory furnace lined with haematite which oxidises carbon to carbon monoxide.
Extraction of Aluminum
2Al2O3 + 3C ➡ 4Al + 3CO2
Al2O3 →2 Al³++ 3O²–
At cathode: Al³++ (melt) + 3e- ⟶ Al (l)
At anode: C(s) + O²- (melt) → CO (g) + 2e–
C(s) + 2O²- (melt) ➡ CO2 (g) + 4e–
Graphite rods get burnt forming CO and CO2. The Aluminum thus obtained is refined electrolytically using impure Al as anode, pure Al as cathode and molten cryolite as electrolyte.
(C)Refining: It is the process of converting an impure metal into pure metal depending upon the nature of metal. it is of following types
(1) Distillation (2) Liquation (3) Electrolytic Refining (4) Zone Refining, (5) Vapor phase refining (6) Chromatography
1. Distillation: It is the process used to purify those metals which have low boiling points, e.g., zinc, mercury, sodium, potassium. Impure metal is heated so as to convert it into vapours which changes into pure metal on condensation and is obtained as distillate.
2. Liquation: Those metals which have impurities whose melting points are higher than metal can be purified by this method. In this method, Sn metal can be purified. Tin containing iron as impurities heated on the top of sloping furnace. Tin melts and flows down the sloping surface where iron is left behind and pure tin is obtained.
3. Electrolytic refining: In this method, impure metal is taken as anode, pure metal is taken as cathode, and a soluble salt of metal is used as electrolyte. When electric current is passed, impure metal forms metal ions which are discharged at cathode forming pure metal
At anode: M(Impure) ➡ Mⁿ+ + ne-
At cathode: Mⁿ+ + ne- ⟶ M (Pure)
4. Zone refining: It is based on the principle that impurities are more soluble in the melt than in the solid state of the metal. The impure metal is heated with the help of circular heaters at one end of the rod of impure metal. The molten zone moves forward along with the heater with impurities and reaches the other end and is discarded. Pure metal crystallizes out of the melt. The process is repeated several times and heater is moved in the same direction. It is used for purifying semiconductors like B, Ge, Si, Ga and In.
5. Vapour phase refining:
(a) Mond’s process. Nickel, when heated in stream of carbon monoxide forms volatile Ni(CO)4 which on further subjecting to higher temperature decomposes to give pure metal.
Ni (Impure) + 4 CO ⟶ Ni(CO)4 at 330 350K
Ni(CO)4 ⟶ Ni (Pure) + 4CO at 450-470K
(b) van- Arkel method: It is used to get ultra pure metals. Zr and Ti are purified by this process. Zr or Ti are heated in iodine vapours at about 870 K to form volatile ZrI4 or TiI4 which are heated over tungsten filament at 1800K to give pure Zr or Ti
Ti + 2I2 ⟶TiI4
TiI4 ⟶ Ti + 2I2
Zr(Impure) + 2I2 → + ZrI4
ZrI4 ⟶ Zr(Pure) + 2I2
6. Chromatographic method: It is based on the principle of separation or purification by chromatography which is based on differential adsorption on an adsorbent. In column chromatography, Al2O3 is used as adsorbent. The mixture to be separated is taken in suitable solvent and applied on the column. They are then eluted out with suitable solvent (eluant). The weakly adsorbed component is eluted first. This method is suitable for such elements which are available only in minute quantities and the impurities are not very much different in their chemical behaviour from the element to be purified.